Research
Research Focus
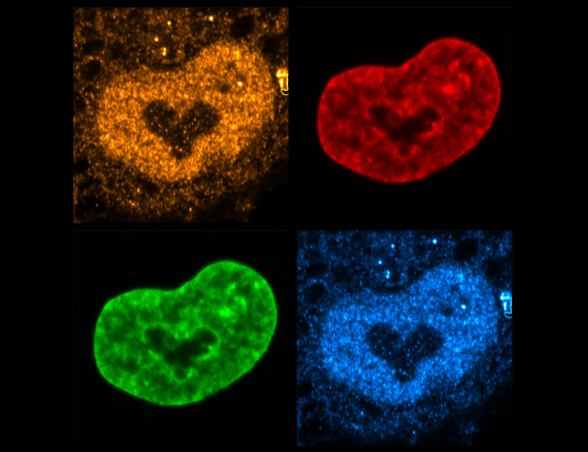
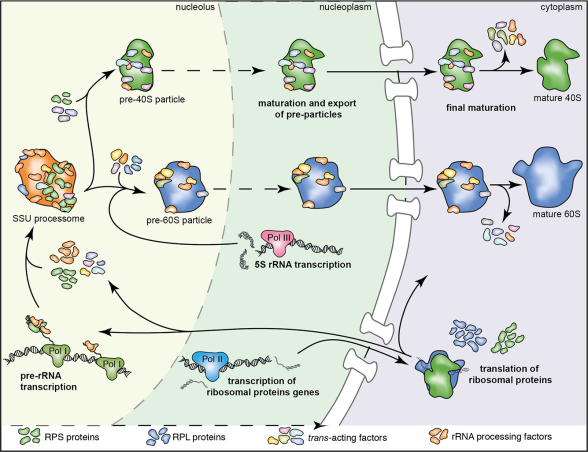
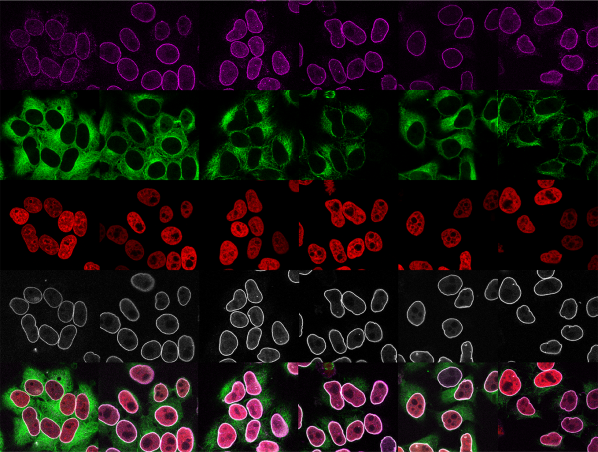
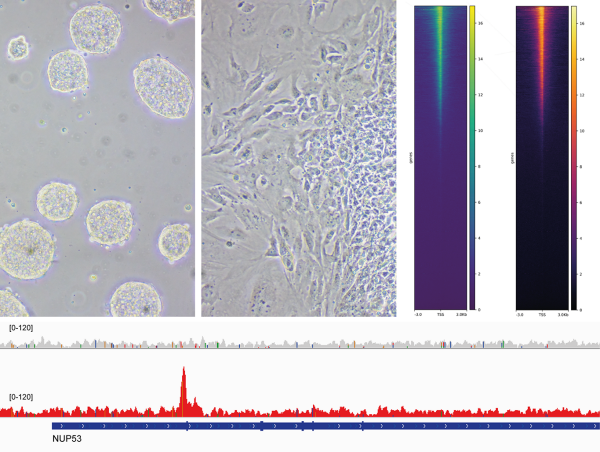
Our laboratory is interested in the organization, function and dynamics of the human cell nucleus. The nucleus is a truly fascinating organelle that is perfectly adapted to its functions as a cellular command center and protective home of our genome. We are investigating different aspects of nuclear biology, including the fundamental biosynthetic function of the nucleus in the assembly of ribosomal subunits, the role of the nuclear envelope in nucleo-cytoplasmic communication and genome organization as well as the dynamics of the nuclear compartment during the cell cycle. By our research, we seek to improve our understanding of how nuclear dysfunction contributes to human diseases such as ribosomopathies, nuclear envelopathies and cancer.
Research topics include: